Lewis-base-catalysed selective reductions of ynones with a mild hydride donor†
Abstract
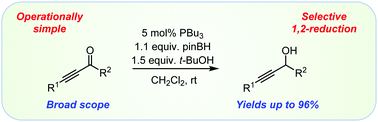
Ynones are efficiently reduced with a mild hydride donor in the presence of a catalytic amount of nucleophilic phosphines. The reactions are selective 1,2-reductions that give propargyl alcohols in yields of up to 96%. It is proposed that success in these reactions depends on the activation of ynones by a Lewis base catalyst. A protic additive plays a key role in suppressing the undesired reaction pathways and accelerating the 1,2-reductions.


 Please wait while we load your content...
Please wait while we load your content...