Aluminium-mediated carbon–carbon coupling of an isonitrile†
Abstract
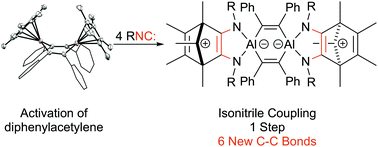
Cp*Al reacts with diphenylacetylene to form a Cp*-substituted 1,4-dialuminacyclohexene. The dialuminacyclohexene reacts with four equivalents of an isonitrile to couple the terminal carbon atoms, forming 6 new carbon–carbon bonds and resulting in a zwitterionic diamide ligand which contains a carbocationic backbone.
- This article is part of the themed collection: Philip Power at 65: an icon of organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...