A reusable cyanide sensor via activation of C–H group: trifluoromethylcarbinol-directed meta-C–H cyanomethylation of naphthalimide†
Abstract
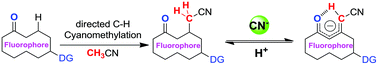
A fluorescent and colorimetric anion sensor based on the activated C–H group has been developed, and its reusability and ability to selectively detect cyanides have been demonstrated. The system utilizes trifluoromethylcarbinol-directed C–H regioselective cyanomethylation for direct C–C bond formation between the fluorophore and acetonitrile. The present paper also describes a novel strategy for the enhancement of carbanion stability by a potential intramolecular C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–C hydrogen bonding system, thus providing a new way to develop activated CH-based anion receptors.
O⋯H–C hydrogen bonding system, thus providing a new way to develop activated CH-based anion receptors.


 Please wait while we load your content...
Please wait while we load your content...