Genotoxicity of ortho-quinones: reactive oxygen species versus covalent modification
Abstract
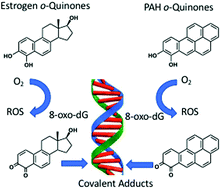
o-Quinones are formed metabolically from natural and synthetic estrogens as well as upon exposure to polycyclic aromatic hydrocarbons (PAH) and contribute to estrogen and PAH carcinogenesis by genotoxic mechanisms. These mechanisms include the production of reactive oxygen species to produce DNA strand breaks and oxidatively damaged nucleobases; and the formation of covalent depurinating and stable DNA adducts. Unrepaired DNA-lesions can lead to mutation in critical growth control genes and cellular transformation. The genotoxicity of the o-quinones is exacerbated by nuclear translocation of estrogen o-quinones by the estrogen receptor and by the nuclear translocation of PAH o-quinones by the aryl hydrocarbon receptor. The properties of o-quinones, their formation and detoxication mechanisms, quinone-mediated DNA lesions and their mutagenic properties support an important role in hormonal and chemical carcinogenesis.


 Please wait while we load your content...
Please wait while we load your content...