Vacuum-evaporated all-inorganic cesium lead bromine perovskites for high-performance light-emitting diodes†
Abstract
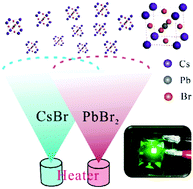
All-inorganic perovskite materials, i.e. cesium lead halide (CsPbX3 (X = I, Br, Cl)), have attracted much attention in the application of photoelectronic devices, especially in solar cells and light-emitting diodes (LEDs). However, the solubility issue of CsPbX3 restricts their utilization in solution-processed devices. Herein, we demonstrated efficient all-inorganic perovskite LEDs (PeLEDs) via co-evaporation of cesium bromide (CsBr) and lead bromide (PbBr2) based on a vacuum thermal evaporation process. The molar ratio of CsBr to PbBr2, which can be adjusted via the co-evaporation ratio, is proved to be very critical to the device performance. Excess CsBr in the perovskite layer causes poor surface morphology and affects the charge transport. With an optimization of the molar ratio, the PeLEDs based on the equimolar CsBr and PbBr2 exhibit the best green electroluminescence (EL) performance with a maximum external quantum efficiency (EQE) of 1.55%. Meanwhile, the full width at half-maximum (FWHM) of the green EL spectrum is as narrow as 18.5 nm, which can offer high color purity and large color space in display applications.


 Please wait while we load your content...
Please wait while we load your content...