Fluoran salicylaldehyde hydrazone Zn(ii) complexes: reversible photochromic systems both in solution and in a solid matrix†
Abstract
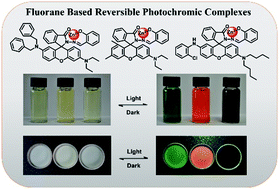
Fluoran dyes have been widely used as chromogenic reagents in piezochromic and thermochromic materials due to their intrinsic halochromic properties. However, reports on fluoran-based photochromic materials are still rare. In this work, a series of fluoran salicylaldehyde hydrazone Zn(II) complexes (1-Zn, 2-Zn and 3-Zn) were facilely prepared. The complexes exhibited excellent reversible photochromism with remarkable fatigue resistance both in solution and in a solid matrix. Due to the multiple colors of the fluoran parts, these complexes exhibited different colors after UV light irradiation. Particularly, a black color was obtained for 3-Zn, which is relatively infrequent because black photochromism systems are still very uncommon. The photochromic mechanism was proposed, where the tautomerism from the enol form to the keto form in the salicylaldehyde hydrazone moiety was induced by UV light irradiation, accompanied by a spirolactam ring-opening reaction and yielding colored products. A smaller group adjacent to the spirolactam ring was favorable for the stability of the colored products. The influences of different metal ions and solvents on the photochromism of these complexes were also studied. Moreover, patterns were successfully visualized in thin layer silica gel that was impregnated with the complexes after UV light irradiation, which suggested that they can be promising candidates for photo-patterning.


 Please wait while we load your content...
Please wait while we load your content...