Dual emission and multi-stimuli-response in iridium(iii) complexes with aggregation-induced enhanced emission: applications for quantitative CO2 detection†
Abstract
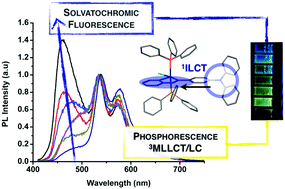
Four new Ir(III) complexes with the general formula [IrHCl(C^N)(PPh3)2] containing different conjugated Schiff base ligands (C^N) have been synthesized and characterized by 1H, 13C, and 31P NMR, HRMS, and IR spectra and one of them by single crystal X-ray diffraction. Their photophysical properties in solution and in the solid state have been analyzed and three main practical results have been obtained: (i) a dual fluorescent and phosphorescent emissive complex in solution, (ii) successful acid/base sensing in the solid state and (iii) quantitative CO2 detection. Quantum chemical calculations have been employed to assign the character of the lowest excited states. A plausible explanation for the observed aggregation induced enhanced emission (AIEE) is given, based on the restriction of intramolecular motions due to the effect of intermolecular C–H⋯π and C–H⋯Cl type interactions upon aggregation.


 Please wait while we load your content...
Please wait while we load your content...