Enhancing field-effect mobility and maintaining solid-state emission by incorporating 2,6-diphenyl substitution to 9,10-bis(phenylethynyl)anthracene†
Abstract
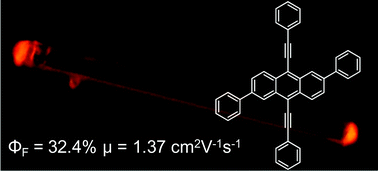
The development of optical and electrical organic semiconductors is crucial for the construction of integrated optoelectronic devices. Herein, a new anthracene derivative, 2,6-diphenyl-9,10-bis(phenylethynyl)anthracene (DP-BPEA), was designed and synthesized by enlarging the π-conjugation of 9,10-bis(phenylethynyl)anthracene (BPEA) via 2,6-diphenyl substitution. Compared with the parent BPEA molecule, an improved field-effect mobility of 1.37 cm2 V−1 s−1 with a comparable solid fluorescence efficiency of 32% is obtained for DP-BPEA, suggesting its potential applications in integrated optoelectronic devices.
- This article is part of the themed collection: Celebrating Excellence in Research: Women of Materials Science


 Please wait while we load your content...
Please wait while we load your content...