Controlling the excited-state energy levels of 9,9′-bifluorenylidene derivatives by twisting their structure to attaining singlet fission character in organic photovoltaics†
Abstract
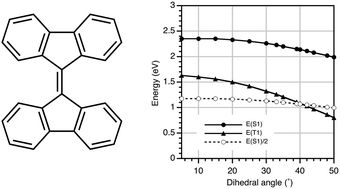
9,9′-Bifluorenylidene (BFN) derivatives, in which the two fluorene moieties are connected via a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond at the 9-position, are expected to show singlet fission (SF) character in organic photovoltaics (OPVs). Due to steric repulsion between the hydrogen atoms at the 1,8- and 1′,8′-positions, the two fluorene planes of BFN are twisted by 31° with respect to each other. DFT calculations suggest that this deviation from coplanarity lowers the T1 energy level of BFN to approximately half that of S1. We synthesized a molecular alkylbithiophene-substituted BFN and a copolymer of diketopyrrolopyrrole and BFN. The optoelectronic properties of these compounds were investigated, and OPV devices using these BFNs as a p-type material were fabricated. The device composed of the polymeric BFN derivative achieved a power conversion efficiency of 4.9%. The observed negative dependence of the photocurrent on the magnetic field suggested that the triplet excitons of the molecular BFN derivatives contribute to the photocurrent in these OPV devices.
C double bond at the 9-position, are expected to show singlet fission (SF) character in organic photovoltaics (OPVs). Due to steric repulsion between the hydrogen atoms at the 1,8- and 1′,8′-positions, the two fluorene planes of BFN are twisted by 31° with respect to each other. DFT calculations suggest that this deviation from coplanarity lowers the T1 energy level of BFN to approximately half that of S1. We synthesized a molecular alkylbithiophene-substituted BFN and a copolymer of diketopyrrolopyrrole and BFN. The optoelectronic properties of these compounds were investigated, and OPV devices using these BFNs as a p-type material were fabricated. The device composed of the polymeric BFN derivative achieved a power conversion efficiency of 4.9%. The observed negative dependence of the photocurrent on the magnetic field suggested that the triplet excitons of the molecular BFN derivatives contribute to the photocurrent in these OPV devices.


 Please wait while we load your content...
Please wait while we load your content...