Mild electropolymerization and monitoring of continuous film formation for photoredox-active Ru metallopolymers†
Abstract
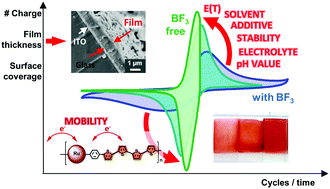
The electropolymerization of a ruthenium(II) 2,6-di(quinolin-8-yl)pyridine complex with two pendant bithienyl-groups is investigated in detail. The associated redox potentials enable mild anodic electropolymerization without the need for boron trifluoride diethyl etherate (BFEE), which promotes undesired side reactions. Continuous film growth in the presence of acid or alternative electrolytes is achieved even for large cycle numbers (500), as detailed by the evolution of the peak current densities and the cathodic charge upon re-reduction of the film. The latter analysis permits a complementary analysis, particularly in the case of distorted CVs caused by charge transport limitations within the film. Notably, electropolymerization can also be performed potentiostatically at low anodic (over-)potentials even at prolonged reaction times, which leads to films with enhanced electrochemical properties. A high film porosity of spherical-like agglomeration networks is confirmed by SEM investigations, whereby higher control is found under slower and/or milder electropolymerization conditions. The films show the preserved electrochemical and optical properties of the Ru sensitizer, i.e., a defined and stable redox process, strong UV-vis absorption up to 600 nm, emission in the NIR range, and effective electrochemical switching promoted by the quaterthiophene bridge.


 Please wait while we load your content...
Please wait while we load your content...