Modular synthesis of asymmetric rylene derivatives†
Abstract
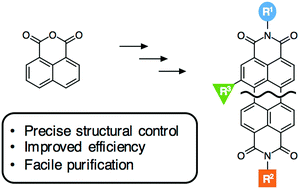
The modular synthesis of asymmetric rylenes from naphthalic anhydride derivatives is presented. Imidization, Suzuki–Miyaura coupling and cyclodehydrogenation reactions are utilized for the generation of novel functional rylenes with these three core transformations providing significant flexibility over the final structure. The combination of simple purification and high yields enables access to asymmetric rylenes with functional handles at the imide-position and site-specific incorporation of bay position substituents. The resulting library of perylenes and bisnapthalimide-anthracene derivatives showcase the presented methodology and the ability to tune optoelectronic and electrochemical properties.


 Please wait while we load your content...
Please wait while we load your content...