Effect of ancillary ligands on the properties of diphenylphosphoryl-substituted cationic Ir(iii) complexes†
Abstract
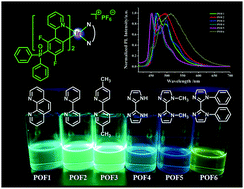
A new class of diphenylphosphoryl-substituted cationic cyclometalated Ir(III) complexes [Ir(POFdFppy)2(N^N)]+PF6− (dFppy = 2-(2,4-difluorophenyl)pyridine) (POF1–POF6) with different N^N ancillary ligands have been synthesized and characterized. The influences of N^N ancillary ligands on the photophysical and electrochemical properties of the Ir(III) complexes have been investigated systematically. The results demonstrate that the photoluminescence quantum yields (ΦPL) of the complexes are dependent on the N^N ancillary ligands. POF1–POF3 equipped with phenanthroline or bipyridine ancillary ligands exhibit intense emission bands at 465–497 nm and high ΦPL in the range of 56–61% in CH2Cl2. The biimidazole-type complexes POF4–POF6 exhibit an obvious substituent effect on the photophysical and electrochemical properties. Although the emission spectra of POF4 and POF5 show similar fine structures, the ΦPL of POF5 bearing two methyl groups at the biimidazole moiety is remarkably lower (5%) than that of POF4 (45%, unmodified biimidazole). POF6 bearing two phenyl groups at the biimidazole moiety exhibits a red-shift and a weak emission band, and an extremely low ΦPL (<3%). However, the photoluminescence quantum yields of POF5 and POF6 (35% and 41% in EC film, 40% and 65% in neat film, respectively) in the film increase effectively in comparison to those in solution. Cyclic voltammetry shows further that the structure of the ancillary ligand affects the redox properties. Density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations were performed to provide insights into the electronic structures of POF1–POF6. POF1 bearing a 1,10-phenanthroline moiety demonstrates the highest oxygen sensitivity.


 Please wait while we load your content...
Please wait while we load your content...