Supramolecular amphiphiles based on cyclodextrin and hydrophobic drugs†
Abstract
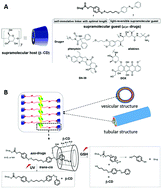
Herein we report a novel “supra-prodrug-type” superamphiphile design: via a redox-sensitive self-immolative linker, a hydrophobic drug molecule was labeled with an azobenzene moiety, which was designed to be capped by a hydrophilic cyclodextrin (CD) molecule. Four clinical hydrophobic drugs, 7-ethyl-10-hydroxycamptothecin (SN-38), doxorubicin (DOX), phenytoin and aliskiren, were investigated to directly participate in building a novel class of superamphiphile, in which the CD moiety plays as the hydrophilic head and the drug as the hydrophobic tail. This novel type of superamphiphile can further self-assemble into vesicular or tubular structures, characterized by transmission electron microscope (TEM), scanning electron microscope (SEM) and dynamic light scattering (DLS). The possible self-assembly mechanism is given based on multiple pieces of evidence, including nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD) and Ultraviolet-visible spectroscopy (UV-vis) results. The reconversion kinetics of the prodrug as a function of glutathione (GSH) in the presence or absence of UV irradiation is presented. Cell experiments indicate that the “supra-prodrug” can be facially endowed with a function by a simple substitution with another functionalized host. We hope this work can provide new reference in the field of drug screening, formulation and delivery.


 Please wait while we load your content...
Please wait while we load your content...