Hierarchical CoS/MoS2 and Co3S4/MoS2/Ni2P nanotubes for efficient electrocatalytic hydrogen evolution in alkaline media†
Abstract
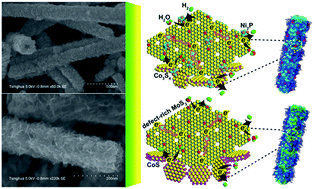
Non-noble metal electrocatalysts have recently received increasing attention for the hydrogen evolution reaction (HER) in acidic electrolytes. However, in alkaline electrolyzers, the slow kinetics of water splitting leads to their poor HER activities. Here, we demonstrate the synthesis of novel hierarchical nanotubes of CoS/MoS2 and Co3S4/MoS2/Ni2P which exhibit prominent and durable HER capabilities in alkaline solution. Particularly, the Co3S4/MoS2/Ni2P nanotubes present an onset overpotential of 60 mV, a Tafel slope of 98 mV dec−1, and a high exchange current density of 0.21 mA cm−2, which are superior to those exhibited by many noble-metal-free electrocatalysts. The enhanced HER properties of the hybrid nanotubes could be related to their open hollow structure, large surface area and active site number, as well as the synergetic cooperation between different components. The results reported here may provide new inspirations for the construction of advanced nanostructures for promising catalytic applications.


 Please wait while we load your content...
Please wait while we load your content...