CO2 derived hydrogen bonding spacer: enhanced toughness, transparency, elongation and non-covalent interactions in epoxy-hydroxyurethane networks†
Abstract
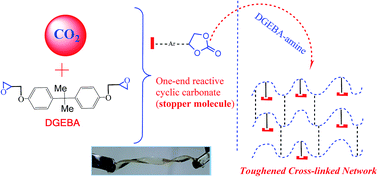
A green and supramolecular approach is demonstrated to enhance the transparency/toughness/adhesive strength of epoxy cross-linked networks. A monocyclic carbonate (MCC) was synthesized by a reaction between CO2 and an epoxy monomer under moderate pressure. By incorporating the one-end reactive MCC into an epoxy network (composed of triamino oligoetheramine and digylcidyl ether bisphenol A), elongation up to 67% was achieved. The epoxy network containing 10 wt% MCC displayed >100% increase in toughness vis-à-vis the pristine network. In addition, two segmental motions were exhibited by the toughened networks, where the lower Tg orients at 55–60 °C and the higher Tg at 73–93 °C. The second Tg is attributed to additional hydrogen bonded regions of the epoxy network (because of the hydrogen bonding spacer unit) as recognized by FTIR studies. Furthermore, studies using density functional theory (DFT) substantiated the existence of two different regions that are responsible for the two Tgs (the second Tg is induced by MCC units via additional hydrogen bonding). The FESEM and AFM investigations further established that no phase separation is present in the networks. The adhesive strengths (LSS) of the epoxy networks increased from ∼17 to 22 MPa due to H-bonding interactions. The networks can perform as self-standing films due to their flexible nature. The networks are transparent (visible transparency increased to >80% by addition of MCC from 68% of the neat epoxy network) and are thermally stable (T10% > 250 °C).


 Please wait while we load your content...
Please wait while we load your content...