Zirconia-supported solid-state electrolytes for high-safety lithium secondary batteries in a wide temperature range†
Abstract
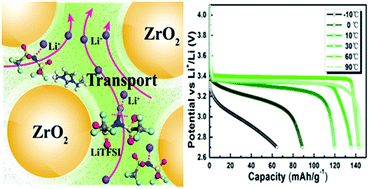
High safety is a long-sought-after goal in the energy storage field. We fabricate a high-safety solid-state electrolyte by in situ immobilizing ionic liquids within a nanoporous zirconia-supported matrix. This ionogel electrolyte provides a combination of the solid-like physical support and liquid-like ionic transport performance, which substantially improves the thermal stability and safety without sacrificing ionic conductivity. Both Raman spectra and density functional theory computations indicate that the zirconia skeleton interacts with the Li salts, promoting the dissociation and transport of Li+. The solid-state cell assembled with this electrolyte possesses excellent cycling performance, with a discharge capacity of 135.9 mA h g−1 after 200 cycles at 30 °C and works well in a wide operating temperature range from −10 to 90 °C. Moreover, the good compatibility and stable interface toward Li–metal anodes in a symmetrical cell demonstrates the usefulness of the electrolyte in Li–metal batteries. These results indicate that this ionogel electrolyte has great promise for application in the energy storage field because of its dramatically improved safety characteristic.


 Please wait while we load your content...
Please wait while we load your content...