Highly selective adsorption of uranium in strong HNO3 media achieved on a phosphonic acid functionalized nanoporous polymer†
Abstract
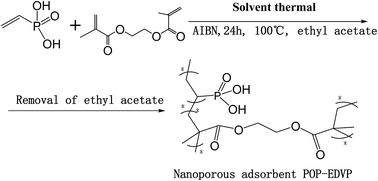
The competition between uranium and other metal ions is one of the greatest challenges for recovery of uranium in strong HNO3 media. In this study, a novel nanoporous organic polymer adsorbent P(EGDMA-VPA) (POP-EDVP) was designed and synthesized by a solvothermal strategy, showing highly selective adsorption of uranium in strong HNO3 media containing 16 co-existing cations. The outstanding selectivity (SU = 94.2%) for the adsorption of uranium on the polymer is reasonably attributed to the presence of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in the polymer skeleton, where the P
O groups in the polymer skeleton, where the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups have a strong complexation with UO2(NO3)2, as evidenced by their XPS results and supported by the density functional theory (DFT) calculation. Very importantly, the polymer shows a maximum adsorption capacity of uranium as high as 215.9 mg g−1 at 298 K in a 4 M HNO3 solution. Even after recycling seven times, there is not any noticeable loss of its sorption capacity. This work shows a simple route to prepare a polymer adsorbent for highly selective adsorption of uranium in strong HNO3 media, which is very significant for recovery of uranium in strongly acidic media.
O groups have a strong complexation with UO2(NO3)2, as evidenced by their XPS results and supported by the density functional theory (DFT) calculation. Very importantly, the polymer shows a maximum adsorption capacity of uranium as high as 215.9 mg g−1 at 298 K in a 4 M HNO3 solution. Even after recycling seven times, there is not any noticeable loss of its sorption capacity. This work shows a simple route to prepare a polymer adsorbent for highly selective adsorption of uranium in strong HNO3 media, which is very significant for recovery of uranium in strongly acidic media.


 Please wait while we load your content...
Please wait while we load your content...