High-energy-density hybrid sol–gel dielectric film capacitors with a polymeric charge blocking layer
Abstract
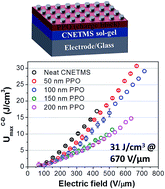
We report dielectric and energy storage characteristics of a bilayer structure based on a hybrid dielectric sol–gel film with a polymeric charge blocking layer. Poly(p-phenylene oxide) (PPO) serves as an interfacial charge blocking layer that suppresses charge injection from a metal electrode into the sol–gel dielectric film based on 2-cyanoethyltrimethoxysilane (CNETMS). The charge blocking layer reduces leakage current in the PPO–CNETMS bilayer film capacitor by more than one order of magnitude, and consequently leads to a maximum discharged energy density of 31 J cm−3 at 670 V μm−1, determined by a charge–discharge measurement. Analysis of the field-dependent polarization reveals that the PPO–CNETMS bilayer films exhibit significantly reduced polarization hysteresis upon charge/discharge cycles, so that the energy extraction efficiency remains higher than that of a neat CNETMS sol–gel film throughout the entire electric field range. We attribute this enhancement to the heightened and widened potential energy barrier at the metal–dielectric interface by the low-loss PPO interlayer. Detailed investigation on the height and width of a potential energy barrier at the metal–dielectric interface, by varying the work function of an electrode (potential energy barrier at zero bias) and the thickness of the PPO layer, indicates the critical role of interface energetics in the energy storage performance of dielectric film capacitors for pulsed power applications.


 Please wait while we load your content...
Please wait while we load your content...