Boosting the energy efficiency and power performance of neutral aqueous organic redox flow batteries†
Abstract
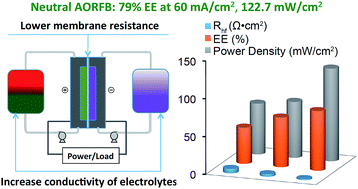
Neutral aqueous organic redox flow batteries (AORFBs) have stood out as a promising RFB technology for sustainable and safe energy storage. It is critical to improve their energy efficiency and power density to meet fast charge/discharge responses in the application of large scale electrochemical energy storage. Herein we show a systematic study on the effects of ion exchange membranes and supporting electrolytes on the resistance and electrochemical performance of a neutral AORFB, the FcNCl/MV AORFB, where FcNCl is (ferrocenylmethyl)trimethylammonium chloride and MV is methyl viologen dichloride. Electrochemical impedance spectroscopic studies revealed that the membrane and the supporting electrolyte are the primary and secondary components accountable for the resistance and charge/discharge overpotential of the neutral AORFB. With an optimized combination of the membrane and the supporting electrolyte, unprecedented energy efficiency and power density of 85% at 40 mA cm−2 and 79% at 60 mA cm−2 and 122.7 mW cm−2 were achieved for the neutral AORFB. The present results emphasize the importance of minimizing battery resistance, and also further advance the promise of neutral AORFBs for sustainable and green energy storage of renewable energy.


 Please wait while we load your content...
Please wait while we load your content...