Tightening polybenzimidazole (PBI) nanostructure via chemical cross-linking for membrane H2/CO2 separation†
Abstract
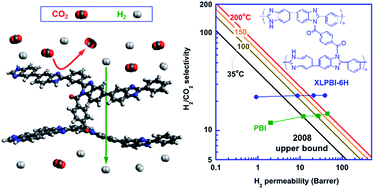
Membranes that permeate H2 and reject CO2 at temperatures above 150 °C are of great interest for low-cost H2 purification and pre-combustion CO2 capture. One of the leading polymers for this separation is poly[2,2′-(m-phenylene)-5,5′-bisbenzimidazole] (PBI), which has good thermal stability and high H2/CO2 selectivity. This study, for the first time, demonstrates that H2/CO2 selectivity can be significantly enhanced by chemical cross-linking of PBI in solid state, in distinct contrast with the literature where cross-linking PBI in solutions decreased H2/CO2 selectivity. We prepared a series of cross-linked PBIs by immersing PBI thin films in terephthaloyl chloride solutions for varying times to achieve different degrees of cross-linking, and then systematically investigated the effect of cross-linking on physical properties including gel content, thermal stability, cross-linking density, fractional free volume (FFV) and inter-chain spacing. Gas sorption and pure- and mixed-gas permeation properties were determined at temperatures ranging from 35 to 200 °C. Cross-linking decreased CO2 sorption and significantly increased H2/CO2 selectivity with a slight decrease in H2 permeability. For example, after cross-linking of PBI, the H2/CO2 selectivity increased from 15 to 23 while the H2 permeability decreased from 45 to 39 Barrers at 200 °C. The performance of this cross-linked PBI surpasses the Robeson's upper bound estimated at 200 °C, indicating its promise for H2 purification and CO2 capture.


 Please wait while we load your content...
Please wait while we load your content...