Structural transformations in Li2MnSiO4: evidence that a Li intercalation material can reversibly cycle through a disordered phase†
Abstract
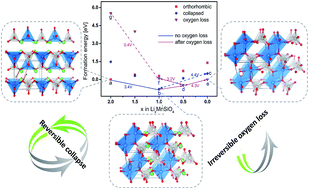
Li2MnSiO4 is a promising high capacity cathode material due to the potential to extract two Li ions per formula unit. In practice, however, the use of Li2MnSiO4 is restricted by a low discharge capacity, which has been attributed to an irreversible structural change in the first charge cycle. In this work, we use density functional theory calculations to explore the details of this structural change, and our results reveal that the structural change during delithiation has two components. First, we find that the material undergoes a structural collapse upon partial delithiation, which is characterized by distortion of the MnO4 tetrahedron. Remarkably, while this transformation results in a disordered structure, our calculations show that it is reversible upon relithiation and that the transformation does not strongly impede Li de/intercalation. The calculated reversibility of the phase change is consistent with recent experimental X-ray diffraction measurements showing that peaks associated with the crystalline MnO4 order, which disappear upon delithiation, are restored upon lithiation. Additional experiments are conducted showing the reversibility of the material during cycling as a function of charging cutoff voltages. Second, we argue that, the irreversible structural degradation is primarily caused by oxygen evolution in the highly delithiated state; the oxygen deficient structure can only reincorporate half of the total Li when discharged to 1.5 V. Experimentally observed voltage profile shifts of Li2MnSiO4 during the first few cycles as well as the different electrochemical behavior exhibited by Li2FeSiO4 can be explained by this two-component structural change model.


 Please wait while we load your content...
Please wait while we load your content...