High efficiency and stable tungsten phosphide cocatalysts for photocatalytic hydrogen production†
Abstract
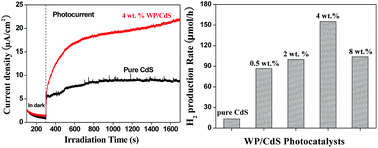
Research and development of high efficiency non-noble metal cocatalysts for solar photocatalytic hydrogen production have been aiming at a long-term goal of a renewable hydrogen economy. This research reports an active and stable tungsten phosphide (WP) cocatalyst as a potential replacement for Pt and Pd noble metal cocatalysts. WP nanoparticles are synthesized via a simple wet chemical process using less expensive and earth abundant materials. The synthesized WP cocatalyst nanoparticles are ball-milled and loaded onto the surface of a CdS photocatalyst, forming a WP/CdS composite. During visible light photocatalytic hydrogen production via the photocatalytic oxidation of an aqueous ammonium sulfite solution, the WP/CdS has shown high photocatalytic activity and stability in comparison with Pt and Pd noble metal cocatalysts. It is found that the 4.0 wt% WP loaded CdS photocatalyst can achieve as high as a 155.2 μmol h−1 hydrogen evolution rate, which is 11.67 times higher than that of the pure CdS photocatalyst and at about 1/3 of the hydrogen production rate (510.4 μmol h−1) of 0.5 wt% Pt loaded Pt/CdS. Electrochemical characterizations of the WP cocatalyst indicate that WP has high electrocatalytic activity and an efficient charge transfer rate between CdS and WP nanocrystals. These are the key factors responsible for the enhanced photocatalytic activity of the WP/CdS photocatalyst.


 Please wait while we load your content...
Please wait while we load your content...