Redox-mediated conversion of atomically dispersed platinum to sub-nanometer particles†
Abstract
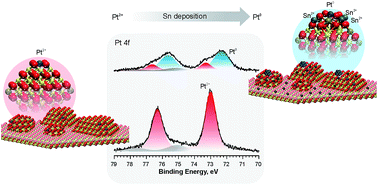
The stability and the conversion of atomically dispersed Pt2+ species to sub-nanometer Pt particles have been investigated as a function of the Sn concentration in Pt–CeO2 films by means of synchrotron radiation photoelectron spectroscopy, resonant photoemission spectroscopy, and angle-resolved X-ray photoelectron spectroscopy in combination with density functional calculations. The deposition of Sn onto the Pt–CeO2 films triggers the reduction of Ce4+ cations to Ce3+ yielding Sn2+ cations. Consecutively, the redox coupling between the Ce3+ and Pt2+ species triggers the reduction of Pt2+ species yielding sub-nanometer Pt particles. The onset of reduction of Pt2+ species is directly related to the concentration of Ce3+ centers which, in turn, is controlled by the concentration of Sn2+ cations in the Pt–CeO2 film. On average, the formation of 6Ce3+ centers corresponding to the adsorption of 3Sn atoms gives rise to the reduction of one Pt2+ species. The analysis of the depth distribution of Sn atoms in the Pt–CeO2 films revealed preferential adsorption of Sn2+ at the surface followed by diffusion of Sn2+ ions into the bulk at higher Sn coverages. Density functional modeling suggested that the adsorption of three Sn atoms in the vicinity of the Pt2+ species results in a rearrangement of the local coordination accompanied by substantial destabilization of the Pt2+ species followed by its conversion to Pt0 atoms. The formation of sub-nanometer Pt particles is coupled with re-oxidation of two Ce3+ centers per one Pt2+ species reduced. Annealing of the Pt–CeO2 films in the presence of metallic Sn also leads to the reduction of the Pt2+ species due to thermally triggered oxidation of metallic Sn residues followed by diffusion of Sn2+ into the bulk. Annealing of the Pt–CeO2 films to temperatures above 600 K results in a loss of Sn yielding sub-nanometer Pt particles supported on nearly stoichiometric and Sn-free CeO2 films.


 Please wait while we load your content...
Please wait while we load your content...