Facilitating hole transfer on electrochemically synthesized p-type CuAlO2 films for efficient solar hydrogen production from water†
Abstract
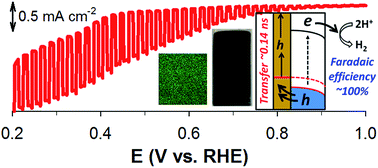
Delafossite CuAlO2 photoelectrodes are synthesized via the electrodeposition of Cu(II) and Al(III) onto fluorine-doped tin oxide (FTO) substrates in water and dimethylsulfoxide (DMSO) solvents, followed by annealing in air and Ar. The surface properties, crystalline structure, and photoelectrochemical (PEC) performance of the as-synthesized samples are significantly affected by the synthetic conditions. Optimized CuAlO2 electrodes (synthesized in DMSO and annealed in air) possess suitable energetics for H2 production under sunlight (an optical bandgap of ∼1.4 eV and a conduction band level of −0.24 VRHE). They exhibit a photocurrent onset potential of ∼+0.9 VRHE along with a faradaic efficiency of ∼70% at +0.3 VRHE in an alkaline solution (1 M KOH) under simulated sunlight (AM 1.5; 100 mW cm−2). The addition of sacrificial hole scavengers (sulfide and sulfite) significantly improves the PEC performance of CuAlO2 by a factor of eight, along with providing a faradaic efficiency of ∼100%. This indicates that the hole transfer limits the overall PEC performance. This issue is addressed by employing a ∼150 nm-thick Au film-coated FTO substrate for the CuAlO2 deposition. In the absence of hole scavengers, the H2 production with the Au-underlain CuAlO2 photoelectrode (Au/CuAlO2) is three-fold higher than that with bare CuAlO2, while the faradaic efficiencies at +0.3 and +0.55 VRHE are ∼100%. The time-resolved photoluminescence emission decay spectra of the CuAlO2 and Au/CuAlO2 confirm the facilitated charge transfer in the latter.


 Please wait while we load your content...
Please wait while we load your content...