Elucidating the origin of oxide ion blocking effects at GDC/SrZr(Y)O3/YSZ interfaces†
Abstract
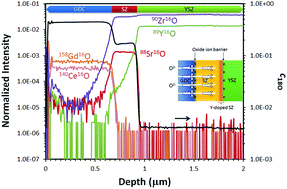
Significant attention has been directed towards the enhancement of the efficiency of solid oxide fuel cells (SOFCs) for energy applications. One of the main issues of an industry-applicable cathode material, (La0.6Sr0.4)(Co0.2Fe0.8)O3−δ, used in conjunction with an yttria-stabilized zirconia (YSZ) electrolyte, is the formation of a SrZrO3 (SZ) phase which results from the chemical reaction between these two materials at high operating temperatures, notwithstanding the utilization of a diffusion barrier layer such as gadolinia-doped ceria (GDC). To gain a better understanding of the effect of the SZ phase on the oxide ion transport through SOFC heterostructures, dense GDC/SZ/YSZ and GDC/SZY(Y-doped SrZrO3)/YSZ multilayer films with well-defined interfaces are prepared using pulsed laser deposition (PLD). Isotope exchange depth profiling with secondary ion mass spectrometry (SIMS) is employed to probe oxide ion transport through the multilayer films, and advanced analytical techniques including scanning/transmission electron microscopy (S/TEM) and spatially resolved electron energy loss spectroscopy (EELS) are used to examine the heterointerfaces. A steep drop in the concentration of the 18O tracer SIMS depth profile reveals the existence of an oxide ion blocking effect occurring at the GDC/SZ(Y) and SZ(Y)/YSZ interfaces. By correlating this result with the EELS analysis of the O K-edge at SZ(Y)/YSZ interfaces, it was found that an interface-induced oxygen disorder possibly arising from Y diffusion across the interface could account for this behavior. Thermodynamic considerations have been made on the construction of plausible phase diagrams for the Sr–Y–Zr–O system to analyze the stability of interfaces. The implications of this interfacial disorder on the performance of SOFCs are discussed.


 Please wait while we load your content...
Please wait while we load your content...