Atomic-scale topochemical preparation of crystalline Fe3+-doped β-Ni(OH)2 for an ultrahigh-rate oxygen evolution reaction†
Abstract
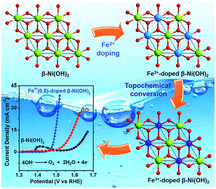
The development of highly efficient and affordable electrocatalysts for the sluggish oxygen evolution reaction (OER) has been considered as a great challenge to the practical applications for water splitting and in rechargeable metal–air batteries. Herein, we report active and robust OER catalysts of Fe3+-doped β-Ni(OH)2 prepared via an atomic-scale topochemical transformation route. Based on the premise that all Fe3+ is incorporated into the β-Ni(OH)2 lattice, the OER activity increases directly with the content of Fe3+. The Fe(0.5)-doped β-Ni(OH)2 catalyst affords a current density of 10 mA cm−2 at an overpotential as low as 0.26 V and a small Tafel slope of 32 mv dec−1. Comparing the state-of-the-art IrO2 catalyst, the Fe(0.5)-doped β-Ni(OH)2 catalyst exhibits higher activity and stability from galvanostatic tests at 10 mA cm−2. Additionally, we experimentally demonstrate that Fe(0.5)-doped β-Ni(OH)2 exerts higher OER activity than Fe(0.5)-doped α-Ni(OH)2. All evidence indicates that Fe and the β-Ni(OH)2 matrix play an important role in NiFe-based catalysts.


 Please wait while we load your content...
Please wait while we load your content...