Stoichiometric water splitting using a p-type Fe2O3 based photocathode with the aid of a multi-heterojunction†
Abstract
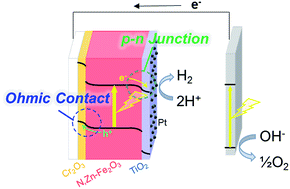
Fe2O3-based photocathodes are one of the least expensive options for hydrogen generation by water splitting. Although p-type N,Zn-doped Fe2O3 (N,Zn–Fe2O3) has been reported to possess a negative conduction band minimum position sufficient for photocathodic hydrogen generation, the efficiency and stability of the resulting H2 production is low and the reaction is sacrificial. In the present work, analysis by hard X-ray photoelectron spectroscopy (HAXPES) showed that these negative characteristics result from the self-redox reaction of p-type Fe2O3. Based on this result, a TiO2 layer was introduced onto the surface of p-type N,Zn–Fe2O3 to passivate surface defects. In addition, to ensure efficient electron transfer, a thin Cr2O3 layer was also inserted between N,Zn–Fe2O3 and a bottom side conductive oxide layer to generate a favorable band alignment for hole transfer. The resulting Pt/TiO2/N,Zn–Fe2O3/Cr2O3 electrode exhibits a highly stable, significantly enhanced cathodic photocurrent during H2 production under AM 1.5 irradiation. The mechanism providing this improvement was investigated by combining electrochemical impedance spectroscopy, open-circuit voltage decay analysis and scanning tunneling electron microscopy-energy dispersive X-ray spectroscopy. Stoichiometric water splitting without an external electrical bias was also demonstrated by connecting the Fe2O3-based photocathode to an n-type SrTiO3−x photoanode, representing the first-ever example of stoichiometric overall water splitting using an Fe-based photocathode.


 Please wait while we load your content...
Please wait while we load your content...