A dual-functional organic p–n bilayer catalyst comprising a perylene derivative and cobalt phthalocyanine working under illumination and in the dark†
Abstract
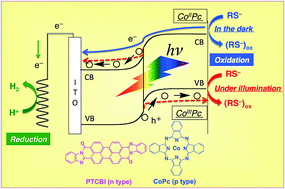
In this study, we show dual-functional catalysis for down-hill reaction by an organic p–n bilayer with and without irradiation. The organic p–n bilayer, composed of 3,4,9,10-perylenetetracarboxylic-bis-benzimidazole (PTCBI, n-type) and cobalt phthalocyanine (CoPc, p-type), is employed as a photocatalyst in the presence of 2-mercaptoethanol. The PTCBI/CoPc bilayer can induce the photocatalytic oxidation of thiol along with the reduction of H+ to H2 by oxidizing and reducing powers generated at CoPc and PTCBI surfaces, respectively, through a series of photophysical events within the organic bilayer. Moreover, the aforementioned reaction can also be found to occur in the dark, due to the catalysis of the bilayer. The distinct oxidation states of CoPc (i.e. CoIIPc in the dark and CoIIIPc under illumination) are responsible for thiol oxidation, where the reducing power for H2 evolution can consist of the electrons released from thiol in the dark and the electron carriers generated under illumination. In this paper, photoelectrochemical and photocatalytic results have been presented to discuss the details of the two types of catalyses by the PTCBI/CoPc bilayer.


 Please wait while we load your content...
Please wait while we load your content...