Improved electrochemical performance of boron-doped carbon-coated lithium titanate as an anode material for sodium-ion batteries†
Abstract
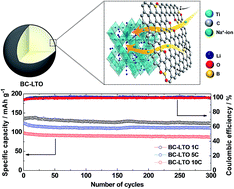
A boron-doped carbon layer (BC) was coated on porous micron-sized Li4Ti5O12 (LTO) via a facile wet-chemical method for use as a promising anode material for sodium-ion batteries. As determined by X-ray photoemission spectroscopy and Raman spectroscopy, three different species (BC3, BC2O, and BCO2) were doped in the carbon layer on the surface of LTO. These heteroatoms lead to abundant extrinsic defects in the carbon layer that result in improved Na-ion diffusivity across the active material. Using electrochemical impedance spectroscopy, the diffusion coefficient of the boron-doped carbon-coated LTO (BC-LTO) was found to be 2.241 × 10−15 cm2 s−1. The BC-LTO exhibited 85% and 60% higher Na-ion diffusivity than pristine LTO and carbon-coated LTO (C-LTO), respectively. Moreover, electron-deficient boron in BC3 enhances the electric conductivity through positively charged holes that carry electrons through the carbon structure. Accordingly, the BC-LTO electrode showed a high specific capacity of 148 mA h g−1 at 0.5C and 98.4 mA h g−1 at 10C rate. In addition, the BC-LTO anode demonstrated a great capacity retention of about 90% at 1 and 10C after 300 cycles. These findings demonstrate the promising potential of LTO as a high-performance anode material for sodium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...