Fe incorporated α-Co(OH)2 nanosheets with remarkably improved activity towards the oxygen evolution reaction†
Abstract
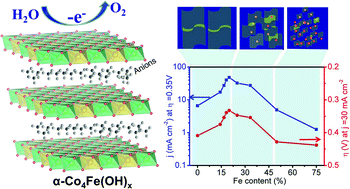
Cost-effective and highly active electrocatalysts for the oxygen evolution reaction (OER) are critical to energy conversion and storage processes. Herein, a superior OER catalyst of Fe substituted α-Co(OH)2 (α-Co1−mFem(OH)2) has been synthesized by taking advantage of the large layered structure and good conductivity of α-Co(OH)2, in conjunction with the rich redox properties and abundance of Fe. The atomically layered α-Co4Fe(OH)x (Co/Fe = 4) nanoplates could effectively catalyze water oxidation at the onset potential of 0.26 V and its turnover frequency (TOF) was 11 and 5 times higher than those of α-Co(OH)2 and IrO2, respectively. The increased activity could be attributed to strong electronic interactions between Co and Fe. Density functional theory (DFT) calculations also demonstrated that the theoretical overpotential of α-Co1−mFem(OH)2 is obviously lower than that of α-Co(OH)2 and thus Fe doped α-Co(OH)2 displays a better activity. Moreover, the correlation between the Fe content and activity could be plotted as a volcano curve.

 Please wait while we load your content...
Please wait while we load your content...