Phosphate modified ceria as a Brønsted acidic/redox multifunctional catalyst†
Abstract
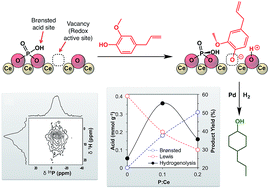
Deposition of trimethylphosphate onto ceria followed by thermal treatment resulted in the formation of surface phosphates with retention of the ceria fluorite structure. The structural and chemical properties of the phosphate-functionalized ceria were studied using 31P solid-state NMR, XPS, zeta titration, ammonia thermal desorption, pyridine adsorption, and model reactions. The introduction of phosphates generated Brønsted acid sites and decreased the number of Lewis acid sites on the surface. The relative amount of Lewis and Brønsted acids can be controlled by the amount of trimethylphosphate used in the synthesis. Upon deposition of Pd, the multifunctional material showed enhanced activity for the hydrogenolysis of eugenol and guaiacol compared to Pd on the unmodified ceria support. This was attributed to the cooperativity between the Lewis acid sites, which activate the substrate for dearomatization, and the redox/Brønsted acid properties, which catalyze hydrogenolysis.


 Please wait while we load your content...
Please wait while we load your content...