A solid oxide photoelectrochemical cell with UV light-driven oxygen storage in mixed conducting electrodes
Abstract
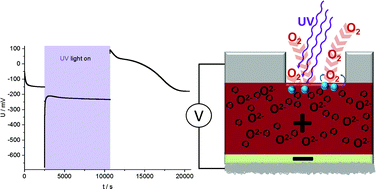
A single crystalline SrTiO3 working electrode in a zirconia-based solid oxide electrochemical cell is illuminated by UV light at temperatures of 360–460 °C. In addition to photovoltaic effects, this leads to the build-up of a battery-type voltage up to more than 300 mV. After switching off UV light, this voltage only slowly decays. It is caused by UV-induced oxygen incorporation into the mixed conducting working electrode and thus by changes of the oxygen stoichiometry δ in SrTiO3−δ under UV illumination. These changes of the oxygen content could be followed in time-dependent voltage measurements and also manifest themselves in time-dependent resistance changes during and after UV illumination. Discharge currents measured after UV illumination reveal that a large fraction of the existing oxygen vacancies in SrTiO3 become filled under UV light. Additional measurements on cells with TiO2 thin film electrodes show the broader applicability of this novel approach for transforming light into chemical energy and thus the feasibility of solid oxide photoelectrochemical cells (SOPECs) in general and of a “light-charged oxygen battery” in particular.


 Please wait while we load your content...
Please wait while we load your content...