Tuning the functional substituent group and guest of metal–organic frameworks in hybrid membranes for improved interface compatibility and proton conduction†
Abstract
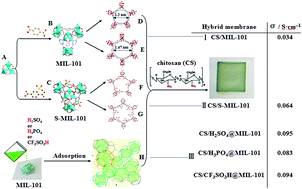
The incorporation of metal–organic frameworks (MOFs) into polymers would be a very promising strategy for overcoming the disadvantage of MOF brittleness and for extending the application of MOFs in proton-conducting materials. Here, we prepare a series of hybrid membranes composed of MOFs and chitosan (CS, very cheap polymer), and systemically study the effect of the incorporation of pure MIL-101 ([Cr3O(H2O)3(bdc)3], bdc = terephthalic acid), the ligand-modified MIL-101, namely S-MIL-101 ([Cr3O(H2O)3(STA)3]·nH2O, STA = 2-sulfoterephthalic acid), and the non-volatile acid-loaded MIL-101, namely acids@MIL-101 (acids = H2SO4, H3PO4 or CF3SO3H), on the interface compatibility and proton conduction of the hybrid membrane. The experimental results revealed the well compatible interfaces between MOF-based materials and the CS matrix because of the hydrogen bond interaction between them, which greatly improved the proton conductivity, activation energy, thermal and mechanical stability, and swelling property of the hybrid membranes. The functionality of MIL-101 is less than that of S-MIL-101 and acids@MIL-101 because of the presence of more hydrogen-bonding sites, proton hopping sites and proton carriers in the latter two types of materials. All MOF-based materials tested (MIL-101, S-MIL-101, and acids@MIL-101) and their hybrid membranes with CS are characterized using field emission scanning electron microscopy (FE-SEM), TEM, EDS, Fourier transform infrared (FT-IR) spectroscopy, thermogravimetric analysis (TGA), and DSC. Fuel cell performances based on these hybrid membranes have been measured. The investigation provides important information for the design of hybrid membranes containing MOFs and polymers.


 Please wait while we load your content...
Please wait while we load your content...