Van't Hoff's law for active suspensions: the role of the solvent chemical potential†
Abstract
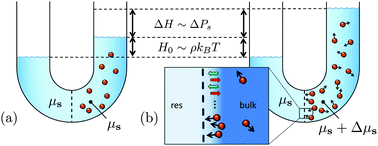
We extend Van’t Hoff's law for the osmotic pressure to a suspension of active Brownian particles. The propelled particles exert a net reaction force on the solvent, and thereby either drive a measurable solvent flow from the connecting solvent reservoir through the semipermeable membrane, or increase the osmotic pressure and cause the suspension to rise to heights as large as micrometers for experimentally realized microswimmers described in the literature. The increase in osmotic pressure is caused by the background solvent being, in contrast to passive suspensions, no longer at the chemical potential of the solvent reservoir. The difference in solvent chemical potentials depends on the colloid–membrane interaction potential, which implies that the osmotic pressure is a state function of a state that itself is influenced by the membrane potential.


 Please wait while we load your content...
Please wait while we load your content...