Vesicles of 2-ketooctanoic acid in water†
Abstract
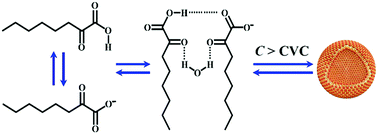
We report the spontaneous formation of vesicles from 2-ketooctanoic acid (KOCOOH), a single-tailed weakly acidic surfactant, in water. The vesicles were characterized using negative-staining, cryogenic transmission electron microscopy, conductivity, and atomic force microscopy. The pH effect on the vesicle formation and the stability of the vesicular structures were determined. The vesicles form at a very low concentration (ca. 1.4 mM) and within a wide pH range (ca. 2–10). Uni- and multilamellar vesicle structures are observed, which coexist in the KOCOOH solution. The hydrogen bonding between KOCOOH molecules probably plays an important role in the formation of the vesicles. Importantly, the vesicles exhibit remarkable stability upon long-term storage, and in artificial seawater. KOCOOH vesicles are a good alternative model system for protocell-like vesicles, as they are easily formed under plausible prebiotic conditions. In addition, they may have the same potential applications, such as in medicine, chemical engineering, and biotechnology, as conventional vesicles. To the best of our knowledge, this is the first report on the vesicles of single-tailed keto-acid amphiphiles.


 Please wait while we load your content...
Please wait while we load your content...