Regimes of electrostatic collapse of a highly charged polyelectrolyte in a poor solvent
Abstract
We perform extensive molecular dynamics simulations of a highly charged, collapsed, flexible polyelectrolyte chain in a poor solvent for the case when the electrostatic interactions, characterized by the reduced Bjerrum length 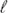 B, are strong. We find the existence of several sub-regimes in the dependence of the gyration radius of the chain Rg on
B, are strong. We find the existence of several sub-regimes in the dependence of the gyration radius of the chain Rg on 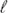 B characterized by Rg ∼
B characterized by Rg ∼ 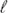 −γB. In contrast to a good solvent, the exponent γ for a poor solvent crucially depends on the size and valency of the counterions. To explain the different sub-regimes, we generalize the existing counterion fluctuation theory by including a more complete account of all possible volume interactions in the free energy of the polyelectrolyte chain. We also show that the presence of condensed counterions modifies the effective attraction among the chain monomers and modulates the sign of the second virial coefficient under poor solvent conditions.
−γB. In contrast to a good solvent, the exponent γ for a poor solvent crucially depends on the size and valency of the counterions. To explain the different sub-regimes, we generalize the existing counterion fluctuation theory by including a more complete account of all possible volume interactions in the free energy of the polyelectrolyte chain. We also show that the presence of condensed counterions modifies the effective attraction among the chain monomers and modulates the sign of the second virial coefficient under poor solvent conditions.



 Please wait while we load your content...
Please wait while we load your content...