High-voltage aqueous supercapacitors based on NaTFSI†
Abstract
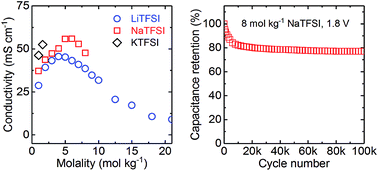
Ultra-high salt concentration has recently been reported to extend the kinetic stability of aqueous electrolytes up to 3 V. However, the low ionic conductivity of these systems makes them unsuitable for high power devices such as supercapacitors. In this study, an 8 mol kg−1 NaTFSI aqueous electrolyte is reported that displays a high stability of 1.8 V on activated carbon during a stringent stability test and a conductivity of 48 mS cm−1 at 20 °C, the latter being comparable to the one of state-of-the-art acetonitrile-based non-aqueous electrolytes. A 1.8 V carbon/carbon supercapacitor employing 8 mol kg−1 NaTFSI displays a high maximum energy density of 14.4 W h kg−1 on the activated carbon mass level and stable cycling for 100 000 cycles. By addition of the redox additive potassium iodide to the electrolyte, the maximum specific energy could be increased to an extremely high value of 37.8 W h kg−1, comparable to the performance of the current generation of commercial non-aqueous supercapacitors.


 Please wait while we load your content...
Please wait while we load your content...