Investigation of polyacrylamide based hydroxide ion-conducting electrolyte and its application in all-solid electrochemical capacitors†
Abstract
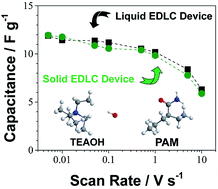
A new hydroxide (OH−) ion-conducting polymer electrolyte comprised of tetraethylammonium hydroxide (TEAOH) and polyacrylamide (PAM) was developed. This electrolyte exhibits excellent ionic conductivity greater than 10 mS cm−1 at room temperature and stable shelf-life over an 80 day exposure in various environments. Solid electrochemical double layer capacitors (EDLC) were fabricated and compared to their liquid counterparts. While all EDLC devices showed similar capacitance, the solid EDLC devices outperformed the liquid devices in cycle-life and offered additional advantages such as safety, light weight, and a flexible form factor. Based on structural, thermal, and chemical analyses, this excellent performance can be attributed to a stable amorphous structure and the higher degree of hydration of the polymer electrolyte promoted by a slight hydrolysis between TEAOH and PAM.


 Please wait while we load your content...
Please wait while we load your content...