Highly active, durable and pH-universal hybrid oxide nanocrystals for efficient oxygen evolution†
Abstract
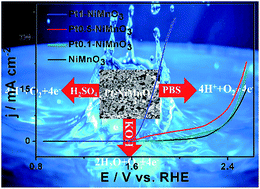
The development of an active, durable, and low-cost catalyst remains hitherto a grand challenge in energy conversion technologies. Herein, hybrid Ptx–NiMnO3 nanocrystals (NCs) (x = 0.1, 0.5, and 1.0 mM) were synthesized by a modified citrate-gel approach and utilized as efficient catalysts towards the oxygen evolution reaction (OER). The results showed that the size of Ptx–NiMnO3 NCs decreases with increasing the initial concentration of Pt. Meanwhile, the OER activity and durability of the as-synthesized Pt–NiMnO3 under different pH conditions are found to be Pt-content-dependent. Particularly, Pt1–NiMnO3 showed superior OER efficiency relative to its counterparts Pt0.5–NiMnO3, Pt0.1–NiMnO3, NiMnO3, and commercial Pt/C at different pH values, ascribed to the size, morphology, and composition effects as confirmed via XRD, XPS and electrochemical characterization.


 Please wait while we load your content...
Please wait while we load your content...