Investigation of the reaction mechanism of lithium sulfur batteries in different electrolyte systems by in situ Raman spectroscopy and in situ X-ray diffraction†
Abstract
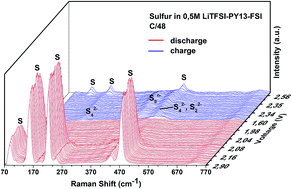
Lithium–sulfur batteries are of great interest owing to their high theoretical capacity of 1675 mA h g−1 and low cost. Their discharge mechanism is complicated and it is still a controversial issue. In the present work, in situ Raman spectroscopy is employed to investigate the poly-sulfide species in the sulfur cathode and in the electrolyte during the cycling of Li–S batteries. The aim is to understand the discharge mechanism and the influence of the electrolyte on the dissolution of sulfur and poly-sulfides. S8n− is identified as the main species in the high voltage plateau of discharge together with cycloocta S8, in the cell using 0.5 mol L−1 LiTFSI–PY13–FSI as the electrolyte. S42−, S22− and S2− are detected soon after the low voltage plateau is reached. A discharge mechanism in the PY13–FSI is proposed based on the identified species which provides important information for improving and designing cathodes. Electrolytes of 0.5 mol L−1 LiTFSI–PY13–FSI and 1 mol L−1 LiTFSI–DOL–DME are used in studying the dissolution of sulfur and poly-sulfides. The results demonstrate that the same poly-sulfide species are present in the two electrolytes. However, the rates of poly-sulfide formation and diffusion to the anode are slow in the ionic liquid compared to those in the ether-based electrolyte due to different ionic mobilities of various species in the two electrolytes. These differences are evidenced by the observation of poly-sulfide species in the DOL–DME from the very beginning of cell assembly even before starting the discharge whereas their appearances, in the ionic liquid, are delayed and only found at the end of the high voltage plateau. Notably, the soluble elemental sulfur is clearly observed in the ionic liquid electrolyte during the first discharge in the high voltage region, which is very different from the DOL–DME system where the elemental sulfur is quickly reduced to poly-sulfides due to self-discharge reactions. In addition, the elemental sulfur is also detected near the lithium anode in DOL–DME at the end of charge, for the first time to our knowledge, which suggests that the degradation of lithium metal is caused by the multiple reactions of the lithium metal surface with soluble poly-sulfides and/or elemental sulfur.


 Please wait while we load your content...
Please wait while we load your content...