Ferrihydrite transformation under the impact of humic acid and Pb: kinetics, nanoscale mechanisms, and implications for C and Pb dynamics†
Abstract
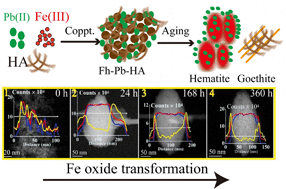
Precipitation of Fe(III) happens very fast across the redox gradient from anoxic to oxic environments and is usually coupled with the co-precipitation of humic acids (HAs) and heavy metals such as Pb. Most previous studies focused on the sorption behaviors of Pb to static phases of Fe oxides or HA. However, little is known about the dynamic interactions between Fe oxides, HA, and Pb. In this study, we presented a systematic, quantitative, and visualized description of ferrihydrite aging and transformation to crystalline Fe oxides under the impact of Pb and HA in abiotic conditions. Wet chemistry experiments were seamlessly complemented by time-resolved chemical imaging with spherical aberration corrected scanning transmission electron microscopy (Cs-STEM) equipped with energy-dispersive X-ray spectroscopy (EDS) and electron energy loss spectroscopy (EELS). Results showed that the presence of HA and Pb slowed down the process of ferrihydrite transformation to hematite. STEM results demonstrated that HA and Pb addition resulted in hematite nanoparticles with a loose and porous structure in comparison with the compact structure of pure hematite nanoparticles. In addition to surface adsorption, EDS mapping and EDS/EELS line scan profiles unambiguously showed that both HA molecules and Pb ions penetrated into the loose, porous structure of hematite nanoparticles, which may act as an important but underappreciated pathway for C and Pb sequestration given the high frequency of Fe oxide transformation in terrestrial ecosystems. Results help to elucidate the environmental behavior of C and Pb during the Fe oxide transformation processes and also shed light on nanoscale mechanisms of organic matter interactions with Fe oxides.


 Please wait while we load your content...
Please wait while we load your content...