Catalytic asymmetric total syntheses of myrtucommuacetalone, myrtucommuacetalone B, and callistrilones A, C, D and E†
Abstract
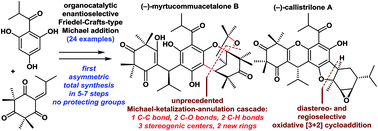
Herein, we describe a concise catalytic approach to the first asymmetric total syntheses of myrtucommuacetalone, myrtucommuacetalone B, and callistrilones A, C, D and E. The syntheses proceed in only 5–7 steps from the readily available compound 11, without the need for protecting groups. Key features of the syntheses include a unique organocatalytic asymmetric Friedel–Crafts-type Michael addition with high enantioselectivity and a broad substrate scope, a novel Michael-ketalization-annulation cascade reaction, and an oxidative [3 + 2] cycloaddition. Furthermore, the new compound 7 exhibited potent antibacterial activities against several multidrug-resistant strains (MRSA, VISA and VRE), and showed greater potency than vancomycin.


 Please wait while we load your content...
Please wait while we load your content...