Z-Selective iridium-catalyzed cross-coupling of allylic carbonates and α-diazo esters†
Abstract
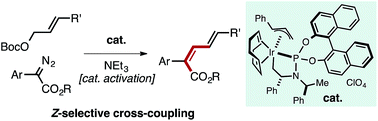
A well-defined Ir–allyl complex catalyzes the Z-selective cross-coupling of allyl carbonates with α-aryl diazo esters. The process overrides the large thermodynamic preference for E-products typically observed in metal-mediated coupling reactions to enable the synthesis of Z,E-dieneoates in good yield with selectivities consistently approaching or greater than 90 : 10. This transformation represents the first productive merger of Ir–carbene and Ir–allyl species, which are commonly encountered intermediates in allylation and cyclopropanation/E–H insertion catalysis. Potentially reactive functional groups (aryl halides, ketones, nitriles, olefins, amines) are tolerated owing to the mildness of reaction conditions. Kinetic analysis of the reaction suggests oxidative addition of the allyl carbonate to an Ir-species is rate-determining. Mechanistic studies uncovered a pathway for catalyst activation mediated by NEt3.


 Please wait while we load your content...
Please wait while we load your content...