Fluoro-substituted cyanine for reliable in vivo labelling of amyloid-β oligomers and neuroprotection against amyloid-β induced toxicity†
Abstract
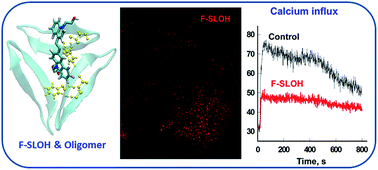
Alzheimer’s disease (AD) is the most prevalent but still incurable neurodegenerative form of dementia. Early diagnosis and intervention are crucial for delaying the onset and progression of the disease. We herein report a novel fluoro-substituted cyanine, F-SLOH, which exhibits good Aβ oligomer selectivity with a high binding affinity, attributed to the synergistic effect of strong π–π stacking and intermolecular CH⋯O and CH⋯F interactions. The selectivity towards the Aβ oligomers in the brain was ascertained by in vitro labelling on tissue sections and in vivo labelling through the systemic administration of F-SLOH in 7 month APP/PS1 double transgenic (Tg) and APP/PS1/Tau triple Tg mouse models. F-SLOH also shows remarkably effective inhibition on Aβ aggregation and highly desirable neuroprotective effects against Aβ-induced toxicities, including the inhibition of ROS production and Ca2+ influx. Its excellent blood–brain barrier (BBB) penetrability and low bio-toxicity further support its tremendous potential as a novel theranostic agent for both early diagnosis and therapy of AD.


 Please wait while we load your content...
Please wait while we load your content...