Hexathioalkyl sumanenes: an electron-donating buckybowl as a building block for supramolecular materials†
Abstract
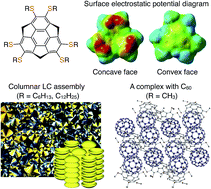
Unlike planar aromatic compounds, bowl-shaped sumanene, which has concave and convex faces with different electrostatic potentials, tends to form a one-dimensional columnar assembly without causing slip-stacking in the crystal. Here we report the first successful synthesis of liquid-crystalline (LC) sumanenes, which was brought about by the incorporation of six thioalkyl groups (R = SC6H13 or SC12H25) into the aromatic part of sumanene. In contrast to the case of the mesophase formation of corannulene, which requires the presence of many dendritic side chains, sumanene derivatives with simple alkyl chains can exhibit a remarkably high-order columnar LC mesophase over a wide temperature range. While non-substituted sumanene inherently behaves as an electron acceptor, hexathioalkyl versions, such as hexathiomethyl sumanene, show electron-donating properties, resulting in complexation with C60. Considering its unique shape, electronic properties, and self-assembly behavior, the electron-donating sumanene may represent a new building block for constructing supramolecular materials, both by itself and in combination with fullerene derivatives.


 Please wait while we load your content...
Please wait while we load your content...