The photochemical alkylation and reduction of heteroarenes†
Abstract
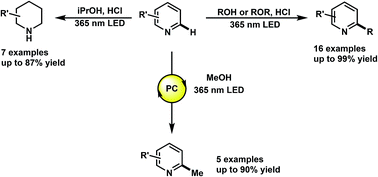
The functionalization of heteroarenes has been integral to the structural diversification of medicinally active molecules such as quinolines, pyridines, and phenanthridines. Electron-deficient heteroarenes are electronically compatible to react with relatively nucleophilic free radicals such as hydroxyalkyl. However, the radical functionalization of such heteroarenes has been marked by the use of transition-metal catalyzed processes that require initiators and stoichiometric oxidants. Herein, we describe the photochemical alkylation of quinolines, pyridines and phenanthridines, where through direct excitation of the protonated heterocycle, alcohols and ethers, such as methanol and THF, can serve as alkylating agents. We also report the discovery of a photochemical reduction of these heteroarenes using only iPrOH and HCl. Mechanistic studies to elucidate the underlying mechanism of these transformations, and preliminary results on catalytic methylations are also reported.


 Please wait while we load your content...
Please wait while we load your content...