A practical and scalable system for heteroaryl amino acid synthesis†
Abstract
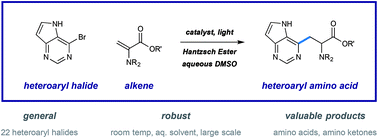
A robust system for the preparation of β-heteroaryl α-amino acid derivatives has been developed using photoredox catalysis. This system operates via regiospecific activation of halogenated pyridines (or other heterocycles) and conjugate addition to dehydroalanine derivatives to deliver a wide range of unnatural amino acids. This process was conducted with good efficiency on large scale, the application of these conditions to amino ketone synthesis is shown, and a simple protocol is given for the preparation of enantioenriched amino acid synthesis, from a number of radical precursors.


 Please wait while we load your content...
Please wait while we load your content...