Thiosemicarbazone organocatalysis: tetrahydropyranylation and 2-deoxygalactosylation reactions and kinetics-based mechanistic investigation†
Abstract
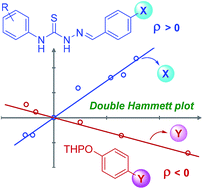
The first use of thiosemicarbazone-based organocatalysis was demonstrated on both tetrahydropyranylation and 2-deoxygalactosylation reactions. The organocatalysts were optimised using kinetics-based selection. The best catalyst outperformed previously reported thiourea catalysts for tetrahydropyranylation by 50-fold. Hammett investigations of both the organocatalyst and the substrate indicate an oxyanion hole-like reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...