Nitro-enabled catalytic enantioselective formal umpolung alkenylation of β-ketoesters†
Abstract
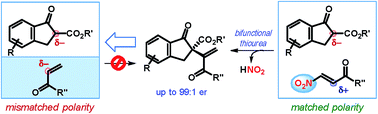
A formal umpolung strategy is presented for the enantioselective installation of an alkenyl group with a terminal double bond at a tertiary center. This one-pot two-step sequence relies on the unique features of the nitro group, which after inverting the polarity of the alkenylating agent toward the desired bond formation, itself serves as a leaving group. The application of this protocol to cyclic β-ketoesters results in densely functionalized products, bearing an all-carbon quaternary stereocenter including an alkenyl substituent with a terminal double bond, in high yields with excellent enantioselectivities.
- This article is part of the themed collection: Celebrating the Chemical Science in India - Leaders in the Field Symposium


 Please wait while we load your content...
Please wait while we load your content...