Discrimination of supramolecular chirality using a protein nanopore†
Abstract
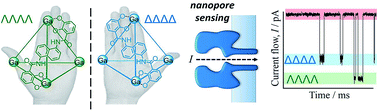
Supramolecular chirality may emerge from self-assembly processes to yield architectures that differ only in the topological arrangement of their constituent parts. Since the properties of the resulting enantiomeric assemblies are identical, purification and characterisation can be challenging. Here, we have examined the hypothesis that the intrinsic chirality of a protein nanopore can be exploited to detect supramolecular chirality. Transient blockages in the ion current flowing through a single membrane-spanning α-haemolysin nanopore were shown to discriminate between M4L6 tetrahedral coordination cages of opposing chiralities. The single-molecule nature of the approach facilitated direct access to the rates of association and dissociation with the nanopore, which allowed the concentrations of the enantiomeric supramolecular assemblies to be determined in situ. Thus, we have established that a protein nanopore can be used to discriminate the chiral topologies of supramolecular assemblies, even when they are too large to fully enter the nanopore.
- This article is part of the themed collection: International Symposium on Macrocyclic & Supramolecular Chemistry (ISMSC) in conjunction with ISACS


 Please wait while we load your content...
Please wait while we load your content...